| |
 |
|
| |
Cyanine dyes exhibit large molar absorptivities (~150,000-250,000M-1cm-1) and moderate quantum yields resulting in extremely bright fluorescence signals. Therefore, cyanines have proven useful in several fields including photography, biology, laser technology and analytical chemistry.
MTTI offers a series of unique lipophilic cyanine dyes which maybe useful for biophysical studies of lipid bilayers of cells or other artifical membranes. In particular, these compounds maybe useful as molecular probes of membrane potential, for labeling lipid bilayers and for labeling hydrophobic pockets of lipo-proteins.
MTTI also offers several substituted indole derivatives which can be used as building blocks for the construction of new cyanine dye derivatives for use in the aforementioned fields.
|
|

|
|
 |
| |
Some Recent Cyanine Dye Publications:
-
Oreopoulos J et al., Combinatorial Microscopy for the Study of Protein-Membrane Interactions in Supported Lipid Bilayers: Order Parameter Measurements By Combined Polarized TIRFM/AFM. J. Struct. Biol., Oct; 168 (1); 21-36 (2009).
-
Oreopoulos J et al., Probing Membrane Order and Topography in Supported Lipid Bilayers by Combined Polarized Total Internal Reflection Fluorescence-Atomic Force Microscopy. Biophys. J. Mar 4; 96 (5): 1970-1984 (2009).
- Bouteiller C et al. Novel Water Soluble Near-Infrared Cyanine Dyes: Synthesis, Spectral Properties, and Use in the Preparation of Internally Quenched Fluorescent Probes. Bioconjugate Chemistry, 18 (4), 1303-1317 (2007).
-
Pham W et al. Synthesis and Applications of a Water Soluble Near Infrared Dye for Cancer Detection Using Optical Imaging. Bioconjugate Chemistry, 16 (3), 735-740 (2005).
-
Hilderbrand SA et al. Monofunctional Near-Infrared Fluorochromes for Imaging Applications. Bioconjugate Chemistry, 16 (5), 1275-1281 (2005).
-
Ye Ye et al. Multivalent Carbocyanine Molecular Probes: Synthesis and Applications. Bioconjugate Chemistry, 16(1), 51-61, (2005)
|
|
Catalog
Number |
Structure/Name |
Formula/MW |
Size* |
Price (USD) |
| CN-1001 |
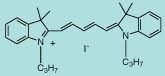
DiI C3(5) |
C31H39IN2
566.6 |
1 mg |
$119.00 |
CN-1002
|
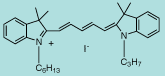
DiI C6,3(5) |
C34H45IN2
608.6 |
1 mg |
$119.00 |
| CN-1003 |
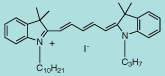
DiI C10,3(5) |
C38H53IN2
664.7 |
1 mg |
$116.00 |
| CN-1004 |
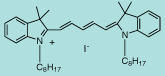
DiI C8(5) |
C41H59IN2
706.8 |
1 mg |
$119.00 |
| CN-1005 |
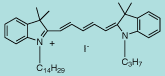
DiI C14,3(5) |
C42H61IN2
720.8 |
1 mg |
$119.00 |
| CN-1006 |
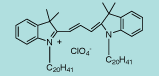
DiI C20(3) |
C60H105ClN2O4
953.9 |
1 mg |
$119.00 |
| CN-1007 |
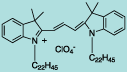
DiI C22(3) |
C64H113ClN2O4
1010.0 |
1 mg |
$119.00 |
| CN-1008 |
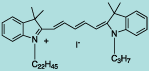
DiI C22,3(5) |
C50H77IN2
833.1 |
1 mg |
$119.00 |
| CN-1009 |
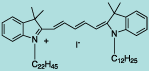
DiI C22,12(5) |
C59H95IN2
959.3 |
1 mg |
$119.00 |
| CN-1010 |
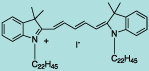
DiI C22(5) |
C69H115IN2
1099.6 |
1 mg |
$119.00 |
| CN-1011 |
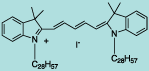
DiI C28(5) |
C81H139IN2
1267.9 |
1 mg |
$119.00 |
| CN-1012 |
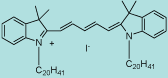
Dil C20 (5) |
C65H107IN2
1043.5 |
1 mg |
$119.00 |
| CN-1013 |
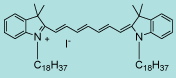
DiR |
C63H101IN2
1013.4
|
10 mg |
$165.00 |
| CN-1014 |
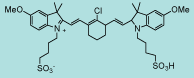
|
C40H51ClN2O8S2
787.4 |
1 mg |
$144.00 |
| CN-1015 |
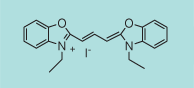
|
C21H21IN2O2
460.3 |
100 mg |
$144.00 |
| CN-1016 |
 |
C51H58N4S3O9
967.2 |
1 mg |
$165.00 |
| CN-1017 |
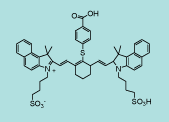
|
C53H56N2S3O8
945.2 |
1 mg |
$144.00 |
| CN-1018 |
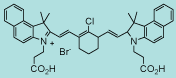 |
C44H44BrClN2O4
780.2 |
1 mg |
$144.00 |
| CN-1019 |
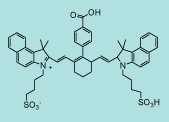
|
C53H56N2O8S2
913.2 |
1 mg |
$144.00 |
| IN-1001 |
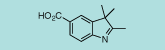
2,3,3-trimethyl-5-carboxy-3H-indole |
C12H13NO2
203.2 |
1 gram |
$170.00 |
| IN-1002 |

2,3,3-trimethyl-5-phthalimidomethyl-3H-indole |
C20H18N2O2
318.4 |
1 gram |
$170.00 |
| IN-1003 |
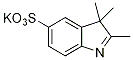
2,3,3-trimethyl-5-sulfo-3H-indole |
C11H13NO3S
239.3 |
1 gram |
$170.00 |
|
| |
* Purity of all compounds >95% |
|
|
 Cyanine Dyes and Cyanine Dye Building Blocks
Cyanine Dyes and Cyanine Dye Building Blocks